Export Procedures In-Shell Pasteurized Shell Eggs to Mexico
January 17, 2024
- Export Procedures - Shell Eggs to Mexico
- LP-210S Online Exhibit
- Disease Free Statement - Pasteurized (January 17, 2024) (pdf)
Ineligible Products
- Consult the Shell Egg Export Restrictions Dashboard prior to certifying any shipments.
- Nest run eggs for breaking facilities are ineligible.
Prerequisites
All Policy and General Procedures Requirements listed in General Export Certification must be met unless otherwise noted in this webpage.
Purpose
This instruction outlines the verification of validated pasteurization processing procedures and specific requirements for the issuance of animal health statements and the shipping certificate required for the export of in-shell pasteurized table eggs to Mexico.
General Procedures
Product Requirements
- Plant management is responsible for providing a Certificate of Compliance (typed on hatchery letterhead, signed and dated) upon request from a USDA representative to demonstrate that the layer flock (identify the company for the source flock) producing the eggs presented for in-shell pasteurization originated from NPIP-monitored breeder flocks.
- Product must originate from layer flocks participating in the NPIP H5/H7 Avian Influenza Monitored Program. Plant management is responsible for identifying all source flocks used for the eggs to be processed. The AMS Grader will verify the source flocks appear on the list of flocks eligible for export: View a listing of commercial table egg layer flocks approved for export (pdf).
- Product must be in compliance with the Food and Drug Administration’s (FDA) regulations for the Prevention of Salmonella Enteritidis in Shell Eggs during Production, Storage, and Transport. Shell eggs originating from a Salmonella positive flock, without additional testing, or shell eggs testing positive for Salmonella may be considered eligible for export provided these eggs are treated and achieve a 5 log reduction of Salmonella.
- The shell eggs intended for pasteurization must be graded and certified at the origin facility, then pasteurized and certified under continuous USDA supervision in an official USDA approved pasteurization facility.
Grade Requirements
Only shell eggs that meet or exceed U.S. Grade A requirements (on-line or stationary lot grading) and are certified as fit for human consumption are eligible for processing and certification as in-shell pasteurized table eggs for export to Mexico. The USDA grader will review the Shell Egg Grading Certificate, Form, LP-210S, accompanying each lot(s) of graded eggs for compliance prior to processing. Additionally, plant management will provide a written procedure describing the measures implemented to maintain the identity of the shell eggs for processing from the time of receipt until processed, packaged, and labeled.
Verification of the Pasteurization Process
The USDA grader will verify the FDA-approved pasteurization process (pasteurization temperature and residence time) for each lot to be certified as pasteurized. These processing parameters may be defined as a range (for example, minimum and maximum temperatures applicable for a defined minimum residence time) to accommodate the in-shell pasteurization of various sizes of eggs. The quality assurance system used shall monitor, on a continuous basis, and maintain records of the processing temperature and the residence time of each lot of pasteurized table eggs. Plant management is responsible for demonstrating continued conformance with the pasteurization treatment and describing the design function of the processing equipment and quality assurance system for the production and packaging of the pasteurized product.
Prior to processing, the USDA grader will conduct a pre-operational inspection to determine that the processing and packaging equipment is clean and in sanitary condition. Any compounds used during the processing and identification of the pasteurized product (cleaning, sanitizing, anti-microbial agents, shell coating, or marking inks) must be USDA accepted for the intended use.
Prevention of Salmonella Enteritidis in Shell Eggs during Production, Storage, and Transport
The FDA regulations state that in-shell pasteurized table eggs are exempt from refrigeration requirements subsequent to packaging. However, when table eggs are refrigerated subsequent to packaging, the refrigeration must be maintained during the transport of the product for export. Therefore, if the U.S. processor elects to refrigerate the in-shell pasteurized table eggs, the grader will monitor the refrigeration (refrigeration temperature defined by the processor) and assure prior to shipment that the transport unit is capable of providing such refrigeration.
Integrity of Product
Each egg must be marked as pasteurized with a distinctive, indelible, and printed mark printed on the shell.
Packaging/Packing
Wooden pallets, constructed with non-manufactured wood material, certified as treated in accordance with the ISPM-15, Phytosanitary Standard as a measure to reduce the risk of introduction or spread of insect larvae or pests and will be branded/stamped with the letters IPPC.
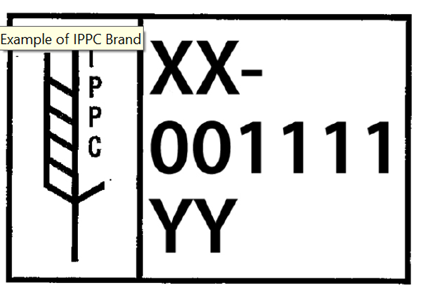
Please Note: Manufactured wooden material, such as compressed particle board, plywood, and corrugated fiberboard are exempt from this standard.
- When IPPC certified pallets show evidence of repair(s), for example, new wood cross members, the pallet or treated wood must be re-certified and branded (IPPC). The original “IPPC” brand may be obliterated and a new brand applied to the pallet. However, for verification purposes, plant management is responsible for maintaining certification documents for all non-manufactured wood material.
- Upon arrival at the processing facility, plant management is responsible for identifying and segregating the new materials, certified non-manufactured wood materials and/or pallets, and advising the USDA grader of the location of the certified materials to be used for export of table eggs to Mexico. The USDA grader will verify that the pallets are certified (IPPC brand) prior to production of a shipment of eggs to be certified for export to Mexico.
Labeling
It is the responsibility of the producer/exporter to assure labeling on retail packaging of in-shell pasteurized table eggs meets in-country requirements.
Packing material (shipping cases) must be identified with the following information in English and Spanish. The context of each proposed label or identification for packing material must also be reviewed by this office for acceptance.
- Keep Refrigerated (when applicable)
- Official Plant Number (USDA assigned plant number)
- Country of Origin
- Statement of Identity (Pasteurized Table Eggs)
- Production Code or Lot Number
- Date of Packaging (Day, Month, and Year)
- Name, address, and mail code of importer (Spanish)
- Name, address, and zip code of exporter (English)
- Expiration or duration date of pasteurized table eggs (as stated by processor)
- The USDA “Graded for Export” stamp including the certificate number
When a label format for either packaging or packing material includes the “Produced From” or the “Verified Pasteurized” grademark, the label must be approved by the National Shell Egg office and assigned an approval number. Further, the shell eggs presented for the production of the pasteurized table eggs must be certified as meeting the quality standards for the grade identified in the shield on the pasteurized product label.
Duration of Quality (Expiration Dating)
If any expiration dating is to be listed on the LP-210s, it must be accompanied by the following statement.
“The expiration date declared on the product is the responsibility of the applicant.”
Certification
When completing the certificate (Form LP-210S) the following information must be included:
- The statement “Certified for Export”.
- The statement “Product of USA”.
- The statement “Fit for human consumption”.
- Production Date(s)
- Packing Date(s)
- Expiration Date(s) as stated by applicant
- Transport Unit seal number(s): ____________


